
Report Adverse Reaction

Approved fees Schedules

Products Recall And Alert

For Stakeholders

Submit a Complaint

FAQs

Submit Advert Application

Submit Advert Application

Consumer Education
News & Events
Latest News
2024-08-12 00:00:00
ONLINE PURCHASE OF MEDICAL DEVICES FOR PERSONAL USE2024-04-17 00:00:00
Ghana's COVID-19 Safety Monitoring Effects Receive Global Acclaim2024-04-05 00:00:00
FDA AND AFROCET MONTGOMERY MEETS STAKEHOLDERS2024-03-13 00:00:00
NEW FDA FEES AND CHARGES EFFECTIVE MONDAY 18th MARCH 20242024-02-01 00:00:00
DRAFT GUIDELINE FOR PUBLIC CONSULTATION "FDA GUIDELINE ON IDENTIFICATION AND DATA CAPTURE FOR PHARMACEUTICAL TRACEABILITY"2023-11-17 00:00:00
JOHN OWUSU GYAPONG IS THE NEXT ARUA SECRETARY-GENERAL2023-11-17 00:00:00
FDA LAUNCHES THE NATIONAL TOBACCO CONTROL STRATEGYUPDATE NO. 6 ON THE SAFETY MONITORING OF THE MALARIA VACCINE
Background
This update summarizes adverse event following immunization reports received between May 2019 and May 2021 from the Malaria Vaccine Implementation Programme (MVIP).
The Ministry of Health launched the first malaria vaccine (Mosquirix or RTS,S/AS01) on 30th April 2019 to be given to young children in routine immunization programme as a complementary malaria control tool that could be added to (and not replace) the core package of WHO-recommended preventive, diagnostic and treatment measures.
In Ghana, the MVIP is taking place in seven regions, namely, Ahafo, Bono, Bono East, Central, Oti, Volta and Upper East Regions of Ghana. Other African countries taking part in the MVIP are Kenya and Malawi.
The pilot is expected to end by 2022 after which a policy decision is required on the potential wider scale use of the vaccine.
What is Adverse Event Following Immunization (AEFI)?
The World Health Organization (WHO) defines an AEFI as any untoward medical occurrence which follows immunization and which does not necessarily have any causal relationship with the usage of the vaccine. Before the launch of the Malaria Vaccine Implementation Programme (MVIP), healthcare workers were trained to report all AEFIs in children who receive Mosquirix to the FDA.
Update on safety monitoring
Review of AEFI reports by the Joint Malaria Vaccine Committee
AEFI reports received in Ghana are reviewed by a seven-member Safety Committee of independent experts, known as the Joint Malaria Vaccine Committee (JMVC).
During this reporting period (May 2019 to May 2021) a total of 731,272 doses of Mosquirix have been given with 2,024 AEFI reports received. This gives a reporting rate of about 28 AEFI reports per 10,000 vaccinated. Out of the 2,024 AEFI reports, 1,964 (97.1%) were received from the phase 4 study (EPI-MAL-003) in the Upper East and Bono East regions where children who received the vaccine are actively followed up and all events after vaccination are documented.
Report by source
*GSK Phase IV study sites (EPI-MAL-003) - 1,964(97.0%)
*Spontaneous reporting (GHS/FDA) - 32(1.6%)
*Malaria Vaccine Pilot Evaluation (MVPE) - 28(1.4%)
The monthly distribution of AEFI reports received from May 2019 to May 2021 is presented in Figure 1.
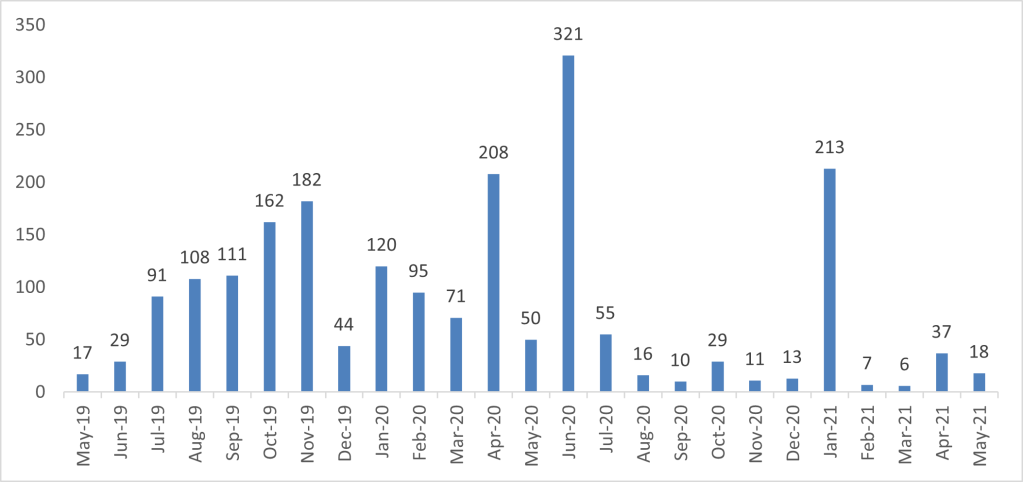
Fig. 1: Number of AEFI reports received by month from May 2019 to May 2021
Out of the 2,024 AEFI reports received, 283 (14%) were serious and the remaining 1,741 (86%) were non-serious. Causality assessment by the Joint Malaria Vaccine Committee of the 283 serious AEFI reports showed that there was no direct relationship between the vaccine and AEFIs reported with the exception of febrile convulsions, fever, gastroenteritis and allergic reaction which were listed in the product information.
Demographic characteristics of persons who reported adverse events
Report by gender
The details on those who reported AEFIs were:
*Males - 1,049(51.8%)
*Female - 925 (45.7%)
*Not indicated - 50 (2.5%)
It is unknown the total number of males and females vaccinated because this information is not routinely collected.
Review of Safety Reports by the Data Safety and Monitoring Board
In order to safeguard the well-being of children participating in the MVIP, a seven-member Programme-specific Data Safety and Monitoring Board (DSMB) was set up by the World Health Organization which also regularly review the safety data from the three countries in order to identify, assess causality and monitor any accumulating safety signals.
The first quarter meeting of the DSMB was held on 2nd March 2021, the DSMB concluded, after the review of the safety data, that there were no significant safety concerns which negatively affect the benefit-risk profile of the vaccine. The DSMB therefore, recommended that the MVIP should continue.
THE FDA MISSION
The FDA exist to ensure the safety, quality and efficacy of human and veterinary drugs, food, biological products, cosmetics, medical devices, household chemical substances and clinical trials, and the control of tobacco products through the enforcement of relevant standards to protect public health.

Subscription Management Centre












