12th August 2024
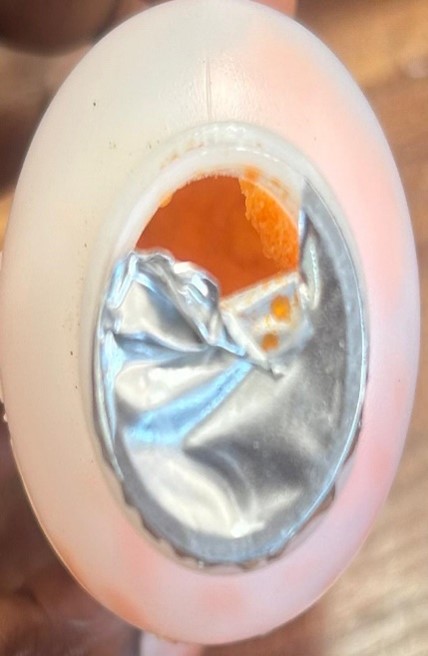
Procold Suspension Drug
Fredun Pharmaceuticals Limited India
Kojach Limited
BE248, BE249, BE250, BE251, BE252, BE253
May 2022
April 2025
This recall is necessitated as a result of warehouse market surveillance conducted at the warehouse. Actual product specification is a powder for oral suspension. The affected batches were caked and not free flowing. This makes the product substandard and therefore not recommended for its intended use.