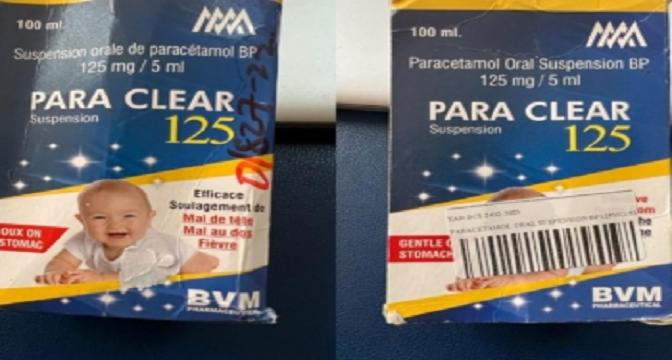
The Food and Drugs Authority (FDA) is notifying healthcare professionals and the public of a substandard paracetamol suspension PARA CLEAR SUSPENSION 125, (Paracetamol Oral Suspension BP 125mg / 5ml) manufactured by SyneCare Mumbai –India.
A report by the National Agency for Food and Drug Administration and Control, Nigeria (NAFDAC) indicated that the product received from the Liberian Medicines and Health Products Regulatory Authority (LMHRA) for laboratory analysis in the NAFDAC Central Drug Control Laboratory (CDCL) confirmed that the product contains ethylene glycol, a toxic substance that is not expected in pharmaceutical formulations.
Para Clear Suspension 125 also failed the requirement for acute oral toxicity with five deaths of the laboratory animals recorded. Ethylene glycol is toxic to humans when consumed and can prove fatal. Toxic effects include abdominal pain, vomiting, diarrhoea, inability to pass urine, headache, altered mental state, and acute kidney injury which may lead to death. Paracetamol suspension is used in children for the treatment of mild to moderate aches and pain, including teething pain, it is also used for the reduction of fever and as an adjunctive treatment to relieve symptoms of cold and flu.
The product is not registered in Ghana by the Food and Drugs Authority and is not expected on the Ghanaian market, however, they may have been distributed illegally. Importers, distributors, retailers and consumers are advised to exercise caution and vigilance within the supply chain to avoid the importation, distribution, sale and use of the substandard (contaminated) syrups. All medical products must be obtained from authorized/licensed suppliers. The products’ authenticity and physical condition should be carefully checked. Anyone in possession of the above-mentioned product is advised to discontinue sale or use and submit stock to the nearest FDA office.
The FDA is encouraging healthcare professionals and the general public to report availability of the substandard syrup on the Ghanaian market to FDA through the hotline 0551112224/5.
You are also requested to report adverse reactions to all products including lack of efficacy, medication error or suspected counterfeit and substandard products to the FDA through the following:
Download and complete the Med Safety App (Google Play Store or App Store)
Complete and submit the report online at http:/adr.fdaghana.gov.gh/
Download and complete the Adverse Reaction (AR) Reporting Form (http://www.fdaghana.gov.gh/operational-guide.php)
Community Pharmacies Designated as Patient Safety Centres
Product details:
The details of the substandard Paracetamol Suspension are as follows:
Product: Name: PARA CLEAR SUSPENSION 125 Paracetamol Oral
Suspension BP 125mg/5ml
Product Manufacturer : SyneCare Mumbai – India
Batch Number : L220008
Manufacturing date : 01/2022
Expiry date : 12/2024