Accra: January 8, 2026 – The attention of the Food and Drugs Authority (FDA) Ghana has been drawn to media reports concerning a recall of certain Nestlé baby food products in some European countries. The countries include France, Germany, Austria, Denmark, Italy and Sweden. The recall is due to potential contamination with cereulide toxin.
The Authority wishes to inform the public that Ghana is not affected by this voluntary recall. Nestlé Ghana Limited has officially confirmed the following to the FDA:
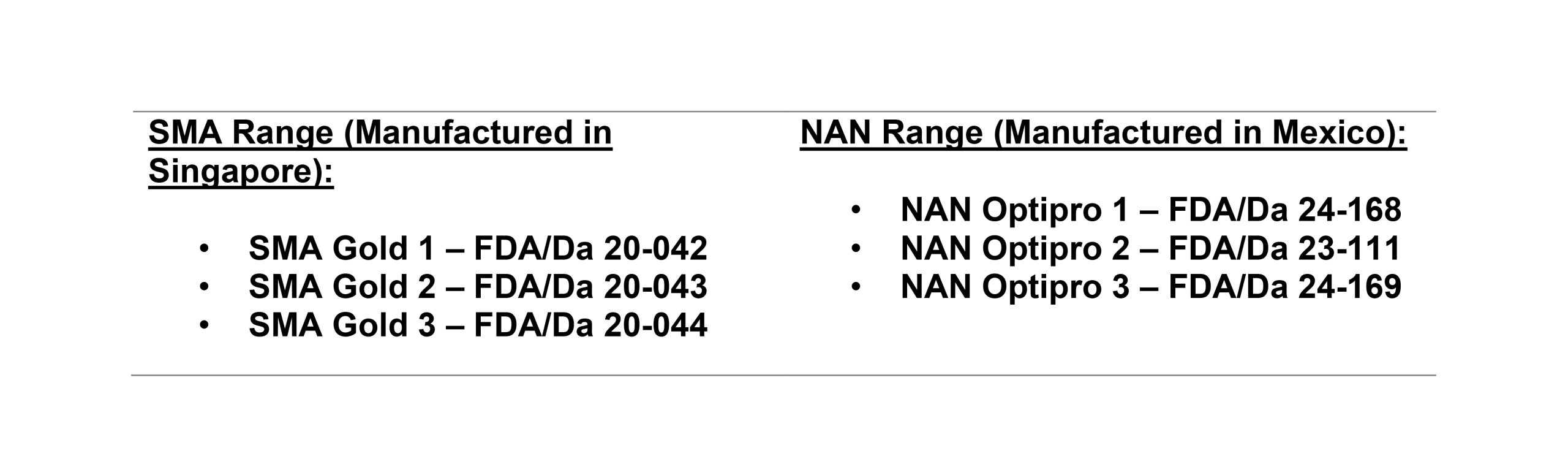
- • SMA infant formula sold in Ghana is manufactured at Nestlé’s Tuas Factory in Singapore (NOT the European facilities affected by the recall)
• NAN infant formula sold in Ghana is manufactured at Nestlé’s Nantli Factory in Mexico (NOT the European facilities affected by the recall)- • All Nestlé infant formula products distributed through Nestlé Ghana’s official channels are duly registered with the FDA and sourced from non-affected manufacturing facilities
• None of the batches subject to the European recall were distributed in Ghana.
FDA-registered Nestlé infant formula products in Ghana are:
While official imports through Nestlé Ghana are confirmed safe, the FDA has taken additional precautionary measures:
- • Requested specific affected batch numbers from Nestlé to enable comprehensive market surveillance
• Deployed inspectors to retail outlets, pharmacies, and distribution points to verify product sources and identify any unofficially imported products
• Enhanced surveillance at all ports of entry to prevent affected batches from entering Ghana through informal channels
UNDERSTANDING CEREULIDE TOXIN RISK
Cereulide is a toxin produced by certain strains of Bacillus cereus bacteria that can cause nausea, vomiting, and stomach cramps. Most importantly, this toxin cannot be destroyed by boiling water during formula preparation, which is why the European recall was initiated as a precautionary measure.
The FDA wishes to inform the general public that all Nestlé infant formula products purchased through legitimate retail channels in Ghana are SAFE to use. These products are manufactured in Singapore and Mexico, not in the European facilities affected by the recall.
Details of the affected batches being recalled can be accessed via: https://www.nestle.com/ask-nestle/products-brands/answers/infant-formula-product-advisory or contact FDA immediately through contacts below.
The FDA remains committed to ensuring the safety of all food products in Ghana, especially those consumed by our most vulnerable population.
Additionally, the public is advised to rely on official FDA communications for accurate information to avoid spreading unverified or misleading reports.





