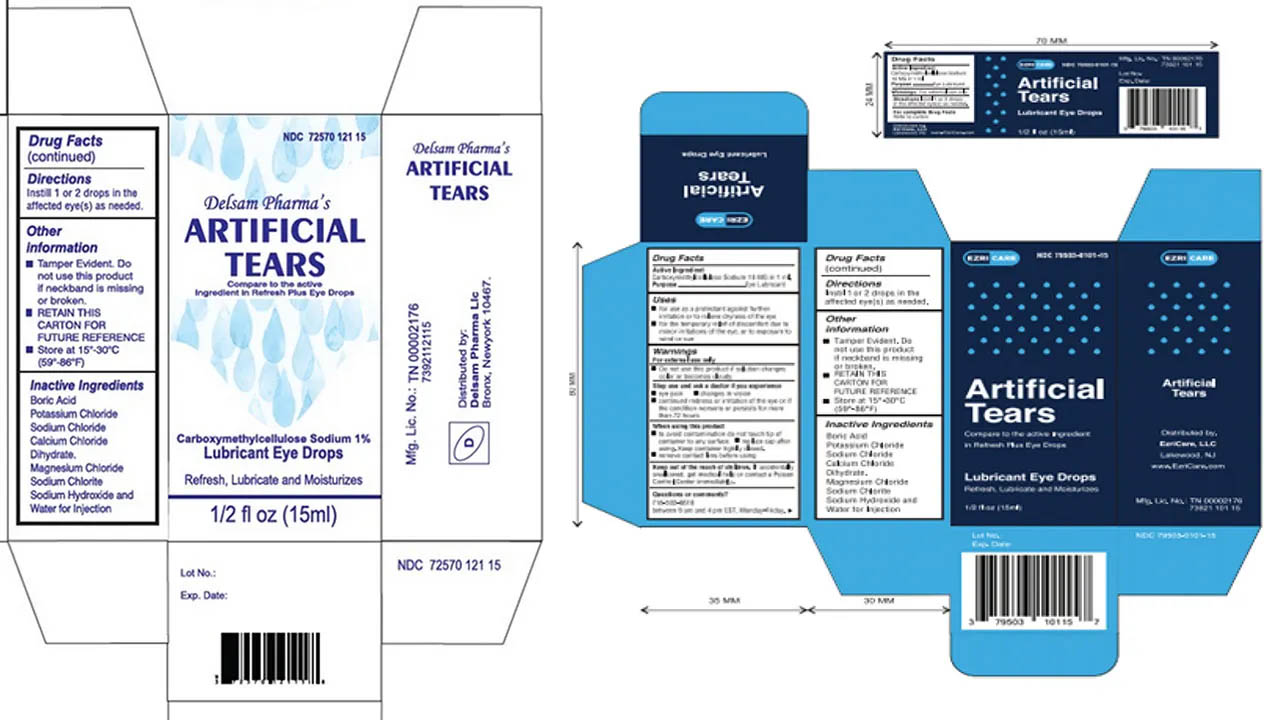
The Food and Drugs Authority (FDA) is alerting the public about a recent major incidence of contamination of two brands of eyedrops, EZRICARE ARTIFICIAL TEARS and DELSAM PHARMA’S ARTIFICIAL TEARS owned by Global Pharma. Global Pharma has since issued a voluntary recall of the products. The US Centre for Disease Control and Prevention (USCDC) has classified the incident as an outbreak. According to the USCDC, the eyedrops were found to contain the drug-resistant bacteria pseudomonas aeruginosa and is suspected to have led to three deaths and vision loss in eight patients. The products are not registered with the Ghana FDA. Therefore, they should not be commercially available on the Ghanaian
market. However, the FDA advises the public who may be in possession of these drugs through other means to immediately stop using the recalled products, Ezricare Artificial Tears and Delsam Pharma’s Artificial Tears, and submit them to any of the FDA offices nationwide.
Anyone who has used these recalled products and is experiencing any symptoms should contact a healthcare professional immediately. Reported symptoms include yellow, green, or clear discharge from the eye, eye pain or discomfort, redness, feeling of something in the eye, increase sensitivity to light and blurred visions. The FDA assures the public that it is taking all necessary measures to ensure that only safe and effective medical products are available in Ghana. The public is encouraged to report any adverse effects or quality problems related to the use of medical products to the FDA through its MEDSAFETY APP.
Below are the images of the recalled products.