
Report Adverse Reaction

Approved fees Schedules

Products Recall And Alert

For Stakeholders

Submit a Complaint

FAQs

Submit Advert Application

Submit Advert Application

Consumer Education
News & Events
Latest News
2024-08-12 00:00:00
ONLINE PURCHASE OF MEDICAL DEVICES FOR PERSONAL USE2024-04-17 00:00:00
Ghana's COVID-19 Safety Monitoring Effects Receive Global Acclaim2024-04-05 00:00:00
FDA AND AFROCET MONTGOMERY MEETS STAKEHOLDERS2024-03-13 00:00:00
NEW FDA FEES AND CHARGES EFFECTIVE MONDAY 18th MARCH 20242024-02-01 00:00:00
DRAFT GUIDELINE FOR PUBLIC CONSULTATION "FDA GUIDELINE ON IDENTIFICATION AND DATA CAPTURE FOR PHARMACEUTICAL TRACEABILITY"2023-11-17 00:00:00
JOHN OWUSU GYAPONG IS THE NEXT ARUA SECRETARY-GENERAL2023-11-17 00:00:00
FDA LAUNCHES THE NATIONAL TOBACCO CONTROL STRATEGYSAFETYWATCH UPDATE No. 02 : COVID-19 VACCINE SAFETY MONITORING WEEKLY UPDATE (9th March to 15th March 2021)
Background
This Update No. 2 provides the safety overview of the COVIID-19 vaccine in Ghana for the period 9th to 15th March 2021. Since publication of Update No. 01 (2th-8th March 2021), which provided the safety overview for the first seven days of vaccine deployment, an additional 161,432 doses of the Covishield Vaccine have been given with 782 persons reporting adverse events (commonly known as side effects) which are mostly mild flu-like symptoms; headache, fever, chills, weakness and body ache.
The Covishield Vaccine continues to be safe and its benefits outweigh possible risks. The reported side effects experienced after vaccination usually resolve within a day or two. Cumulatively the reporting rate is about 3-4 for every 1000 persons vaccinated, which signifies that the FDA has in place, a well-established/robust safety monitoring system.
Highlights
- After 14 days of vaccinations, a total of 424,767 doses of Covishield have been given with 1,489 persons reporting AEFIs; this gives a reporting rate of about 3 to 4 reports per 1,000 doses administered.
- A total of five serious AEFIs reports were received during the period. Three of these were fully investigated with investigations ongoing for the remaining two.
- The Joint COVID-19 Vaccine Safety Review Committee had its second meeting on 12th March 2021.
- The Committee at the second meeting carried out causality assessment for three out of the five serious AEFI reports using the procedure outlined by the World Health Organization and concluded that with the exception of one case of febrile illness (i. e. rapid onset of fever, headache, chills, muscle and joint pains) which is vaccine product related, there was no causal relationship between the other two events and the vaccination.
- The Joint COVID-19 Vaccine Safety Review Committee also discussed reports of blood clots in some countries in Europe following vaccination with the AstraZeneca COVID-19 vaccine, leading to the suspension of vaccination for further investigations and concluded that there is currently no causal link between the event and the vaccine, a position that was subsequently upheld by the European Medicines Agency and the World Health Organization.
Demographic characteristics of persons who reported AEFIs
-Report by gender and age
The details on those who reported AEFIs were:
o Females - 400(56.6%)
o Males - 306 (43.3%)
o Unknown - 1(0.1%)
o Mean age - 41 (SD =14)
AEFI report by monitoring type
- Enhanced spontaneous reporting - 537 (76%)
- Cohort event monitoring - 170 (24%)
Description of AEFIs
The top ten most commonly reported AEFIs based on the number of times these were reported during the two weeks of vaccinations is shown in Figure 1:
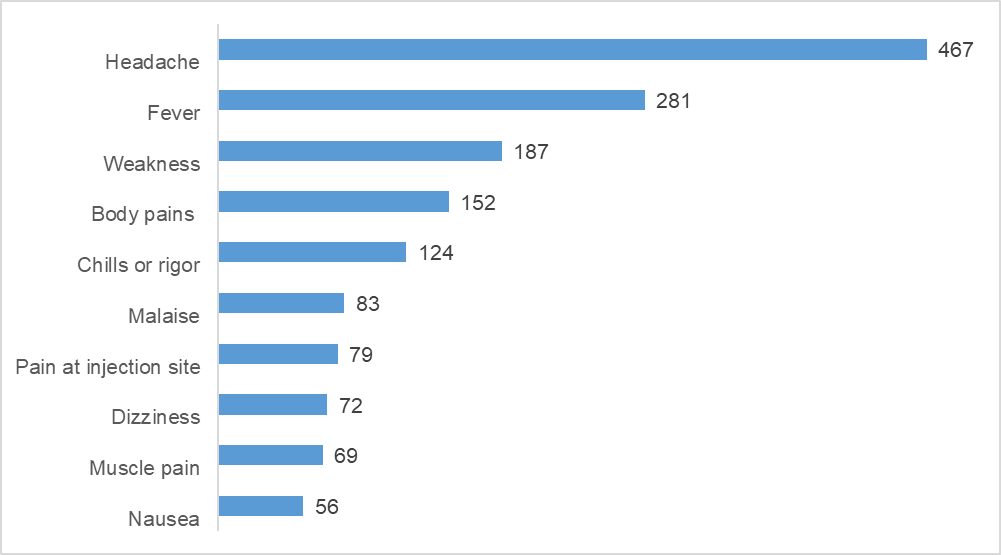
Fig. 1: The top ten most commonly reported events during the two weeks of vaccinations
Assessment of adverse event reports
There might be no relationship between the AEFIs and the vaccine - it may be a coincidence that the adverse events occurred when the vaccine was given.
All adverse event reports received by the FDA are reviewed by the Joint COVID-19 Vaccine Safety Review Committee to find out the possible link between the events and the vaccine.
How to Report AEFIs to COVID-19 vaccines to the FDA
Reporting AEFIs help the FDA have more details about the safety of vaccines to enable any needed regulatory action to be taken to ensure public health and safety.
For any vaccine safety related information or to report AEFIs to COVID-19 vaccines, please contact the FDA through the following:
Mobile:024 4310 297
Email: drug.safety@fda.gov.gh
Hotlines:0551112224/0551112225
Online: http://adr.fdaghana.gov.gh/patient.php
WhatsApp:0551112225
Medsafety App: Download from the Apple store or Google play store
...................................................
1An AEFI is any untoward medical occurrence which follows immunization and which does not necessarily have a causal relationship with the usage of the vaccine.
2An AEFI that results in death, is life-threatening, requires hospitalization or prolongation of existing hospitalization, results in persistent or significant disability or incapacity, or is a birth defect.
3World Health Organization. User Manual for the Revised WHO Classification on Causality Assessment of An Adverse Event Following Immunization (2018). Accessed 15th March 2021. Available from https://apps.who.int/iris/handle/10665/259959
THE FDA MISSION
The FDA exist to ensure the safety, quality and efficacy of human and veterinary drugs, food, biological products, cosmetics, medical devices, household chemical substances and clinical trials, and the control of tobacco products through the enforcement of relevant standards to protect public health.

Subscription Management Centre












